Abstract
Background: Activation of Bruton tyrosine kinase (BTK) is implicated in the pathogenesis of various hematologic malignancies. The BTK inhibitor ibrutinib has activity against other kinases, including interleukin-2-inducible T-cell kinase (ITK), epidermal growth factor receptor (EGFR) and tyrosine kinase expressed in hepatocellular carcinoma (TEC) (Sargiv-Barfi, 2015; Wang, 2016; Chen, 2016), which may contribute to some of its reported toxicities. Acalabrutinib is a highly selective, potent, covalent inhibitor of BTK with minimal off-target activity. The safety profile of acalabrutinib monotherapy was evaluated using pooled safety data from 7 ongoing clinical studies in hematologic malignancies.
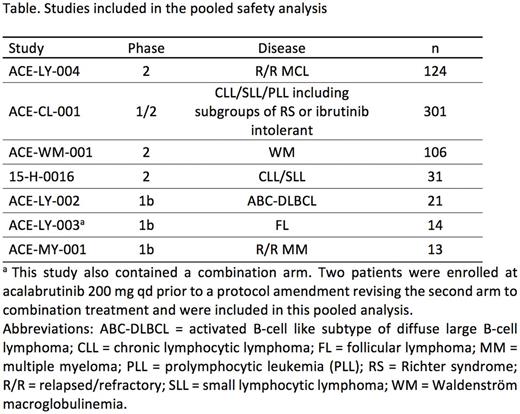
Methods: Safety data from all patients (pts) treated with ≥1 dose of acalabrutinib monotherapy in the studies listed in the Table were pooled for analysis. Acalabrutinib was administered at 100-400 mg daily. Safety data evaluated in the pooled analysis included pt incidence and severity of adverse events (AEs) and rates of treatment discontinuation and dose modifications.
Results: Safety data were pooled from 610 pts. The median age was 66 years (range 32.0-90.0). Baseline ECOG performance status was ≤1 in 95% of pts, and the median number of prior treatments was 1 (range 0-13). The median duration of exposure was 14.2 months (range 0.03-32.4), and the total exposure was 701.5 pt-years. AEs of any grade occurred in 603 (98.9%) pts and treatment-related AEs of any grade occurred in 445 (73%). The most common any-grade AEs (≥20%) were headache (42.3% any cause; 29.2% treatment related; 1.3% Grade ≥3), diarrhea (38.4%; 16.6%; 2.1%), fatigue (23.4%; 7.4%; 1.5%), nausea (23.1%; 9.3%; 1.5%) and contusion (21.6%; 13.4%; 0%). Less than half (n=295 [48.4%]) of all pts experienced Grade ≥3 AEs, the most frequent of which (≥3%) were neutropenia (9.3% any cause; 6.6% treatment related), anemia (7.0%; 1.5%), pneumonia (5.7%; 1.1%) and thrombocytopenia (3.6%; 1.5%). Serious AEs (SAEs) were reported in 218 (35.7%) pts and were considered related to study treatment in 58 pts (9.5%). Grade 5 AEs were reported in 22 (3.6%) pts, the most frequent was pneumonia, occurring in 6/22 pts; 3/22 were considered treatment related by the investigator: esophageal carcinoma, hepatic failure due to hepatitis B reactivation, and intracranial hematoma. Atrial fibrillation (AF) of any grade was reported in 14 (2.3%) pts, of which 6 events (1.0%) were Grade 3; there were no Grade 4 or 5 AF events. Most AF events (12/14) occurred in pts with known risk factors or contributing factors including concurrent infections/pneumonia (2/14), hypertension (4/14), pre-existing cardiovascular disease (4/14) and history of AF (2/14). Hemorrhage Grade ≥3, SAE and/or any grade or seriousness of central nervous system (CNS) hemorrhage was reported in 15 (2.5%) pts, most frequently in the gastrointestinal tract (5/15) and CNS (3/15). Six of these 15 events were SAEs; 4 were treatment related, 1 of which was fatal. Infection events of any grade were reported in 372 (61.0%) pts; Grade ≥3 infection occurred in 99 (16.2%) pts, with pneumonia as the most frequently reported event (n=35 [5.7%]). Opportunistic fungal infections were reported in 4 pts: Pneumocystis jirovecii pneumonia (Grade 2), aspergillosis (Grade 2 and Grade 5), and cryptococcal pneumonia (Grade 2). Five cases (0.8%) of tumor lysis syndrome were reported: 4 cases (1 Grade 4 [treatment related by investigator], 2 Grade 3 and 1 Grade 2) occurred after discontinuing treatment due to disease progression, and 1 case (Grade 3) occurred on day 7 and was considered treatment related by investigator, but did not lead to discontinuation. AEs led to treatment discontinuation in 37 (6.1%) pts. AEs leading to discontinuation in ≥2 pts included pneumonia (0.5%), thrombocytopenia (0.5%), anemia (0.3%), dyspnea (0.3%), glioblastoma multiforme (0.3%), and neutropenia (0.3%). Dose reductions (intake of less than the prescribed dose for ≥3 consecutive days) due to AEs occurred in 12 pts (2.0%). The median relative dose intensity was 98.5%.
Conclusions: Acalabrutinib monotherapy shows a tolerable safety profile across various hematologic malignancies. Of the most common AEs, the majority were low grade (Grades 1-2) and did not lead to treatment discontinuation.
Byrd: Janssen: Research Funding; The Ohio State University: Patents & Royalties: OSU-2S; Pharmacyclics: Research Funding; Acerta Pharma: Research Funding; Genentech: Research Funding. Owen: Acerta Pharma: Consultancy; Pharmacyclics: Consultancy; Janssen: Consultancy, Honoraria, Other: Support to attend academic meetings; Celgene: Consultancy, Honoraria; Takeda: Honoraria, Other: Support to attend academic meetings. O'Brien: Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Acerta: Other: Research Support: Honorarium, Research Funding; Pfizer: Consultancy, Research Funding; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; ProNAI: Other: Research Support: Honorarium, Research Funding; Sunesis: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding; Alexion: Consultancy; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Janssen: Consultancy; Astellas: Consultancy; Vaniam Group LLC: Consultancy; AbbVie: Consultancy; Aptose Biosciences, Inc.: Consultancy; Amgen: Consultancy; GSK: Consultancy. Brown: Gilead: Consultancy, Research Funding; Janssen Oncology: Honoraria; Redx: Consultancy; Astellas Pharma: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; Janssen: Consultancy; Infinity Pharmaceuticals: Consultancy; Pharmacyclics: Consultancy; AbbVie: Consultancy, Honoraria; Celgene: Consultancy; AstraZeneca: Consultancy; Sun BioPharma: Consultancy, Research Funding. Hillmen: Janssen: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; Celgene: Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. Bitman: Astra Zeneca: Equity Ownership; Acerta Pharma: Employment. Chernyukhin: Acerta Pharma: Employment; Astra Zeneca: Equity Ownership. Hamdy: Acerta Pharma: Employment, Equity Ownership, Patents & Royalties: Acalabrutinib related patents. Izumi: Acerta Pharma: Employment, Equity Ownership. Patel: Acerta Pharma: Employment. Schwartz-Sagi: Acerta Pharma: Employment. Tucker: Acerta Pharma: Employment, Equity Ownership; Astra Zeneca: Equity Ownership. Wiestner: Acerta Pharma: Research Funding; Pharmacyclics: Research Funding. Rule: Roche: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Astra-Zeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Kite: Consultancy; Napp: Consultancy; Sunesis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Wang: Pharmacyclics: Consultancy, Honoraria, Research Funding; Acerta Pharma: Consultancy, Honoraria, Research Funding; Kite Pharma: Research Funding; Oncoceutics: Research Funding; BeiGene: Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Asana: Research Funding; Oncternal: Research Funding; Karus: Research Funding; Juno Therapeutic: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal